Hello, my name is Ari Jacobson and I am a rising senior at Northwestern University studying in Integrated Science, Biomedical Engineering and Computer Science. This summer I am working with Dr. H. Christopher Fry under lead scientist Dr. Gary Wiederrecht in the Nanophotonics & Biofunctional Structures group at the Center for Nanoscale Materials at Argonne National Laboratory.
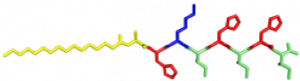
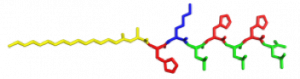
In the Nanophotonics & Biofunctional Structures group many of the projects seek to create functional nanomaterials that are inspired by functions in nature. One of the projects I worked on used peptide amphiphiles (PAs) to bind heme porphyrin and transition metals. This project gets its inspiration from nature, where there exist enzymes which bind heme and copper in order to complete electron transfer and that can exhibit catalytic properties. Our PAs are synthesized by solid phase peptide synthesis using an automated synthesizer. They consist of a peptide sequence capped with palmitic acid at the N-terminus of the . These PAs self-assemble in water, sometimes dependent on pH, and can form supramolecular structures including micelles, ribbons, fibers, and sheets. The peptide sequence at the end can be selected to be bioactive and exposed on the exterior of the nanostructures.
In my project, I compared two peptide sequences and used a variety of spectroscopy techniques to probe heme, copper, and nickel coordination with the amphiphiles. We used PAs with sequences C16-AHKIHIHI-CONH2 (IHIHI PA) and C16-AHKLHLHL-CONH2 (LHLHL PA). These are designed with a histidine towards the hydrophobic region in order to bury a bound heme molecule, and two histidines towards the hydrophilic region to bind copper, nickel, or potentially other transition metals. I hypothesized that using leucine instead of isoleucine in between the histidines would create a more flexible backbone which would increase the efficiency of binding, and binding both heme and a transition metal.
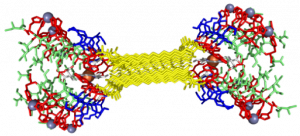
My findings suggested binding of both copper and heme for IHIHI PA. We confirmed heme binding with UV-vis spectroscopy, as we saw a peak at 425 nm when the sample was heated, as we were able to show an effect on the secondary structure of IHIHI PA when copper is added to the solution using circular dichroism (CD), a change in morphology via atomic force microscopy (AFM), and a change in the binding of heme after copper has been added via UV-Vis spectroscopy.
With our LHLHL PA, we were similarly able to show heme binding with heat, showing a peak in UV-Vis at 425 nm. We were not able to see a secondary structure change upon the addition of copper, but we were able to see a change in heme binding when copper was added first, as there was a blue shift in the spectra when more copper was added, eventually showing no heme binding when enough copper was added. This indicates that copper is interacting with our peptide.
From the data I collected this summer using a variety of spectroscopy techniques, we are able to see that copper interacts with both peptides. We are not, however, able to confirm that copper and heme are bound at the same time when heme is added first. In order to further understand this, electron paramagnetic resonance (EPR) spectroscopy must be performed. Once this binding is better understood, it will be possible to further optimize the system and characterize any catalytic or electron transfer capabilities this system may hold.
I am grateful for the opportunity that I had at Argonne National Lab this summer. I would like to recognize the work of Dr. Jennifer Dunn at Northwestern – Argonne institute of Science and Engineering and Dr. H. Christopher Fry at the Center for Nanoscale Materials for their work this summer in making this possible and mentoring me.